Adventure awaits - before they go, consider cholera protection
Why VAXCHORA?1
Indication and usage
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera-affected areas.
Limitations of Use: The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
The Advisory Committee on Immunization Practices (ACIP) recommends VAXCHORA for people 2 through 64 years of age who are traveling to an area with active cholera transmission.2*
To read the full recommendation and see the most recent updates, visit the CDC website.
Read CDC Recommendation Here*The CDC considers cholera transmission to be active in a country when any of the following criteria are met:
-
• WHO reports a cholera outbreak (e.g., annual reports, outbreak bulletins, disease outbreak news)
• A country reports more than 100 suspected cholera cases in the previous 12 months as documented by reliable, publicly available sources such as WHO or the Ministries of Health
• CDC experts have reviewed available information and have determined that cholera is likely present even if not officially reported
CDC, Centers for Disease Control and Prevention; WHO, World Health Organization.
Demonstrated Efficacy Profile1
Efficacy in adults
Study 2 — Challenge Study in Adults Aged 18-45
Study Design: VAXCHORA was studied in a randomized, double-blind, saline placebo–controlled V. cholerae challenge study conducted in U.S. subjects 18 through 45 years of age (N=197) with no prior history of cholera infection or travel to a cholera-endemic area in the previous 5 years were randomized according to a 1:1 ratio to receive 1 dose of VAXCHORA or placebo. The co-primary objectives were to demonstrate the efficacy of a single dose of VAXCHORA in the prevention of moderate to severe diarrhea following challenge at 10 days and 3 months post-vaccination.1,3,a,b
Pre-specified criteria for success were that the lower bound of the two-sided 95% confidence interval for vaccine efficacy must be ≥30% in both the 10 Day and 3 Month challenge groups.1
VAXCHORA demonstrated vaccine efficacy against the occurrence of moderate to severe diarrhea following challenge with V. cholerae O1 El Tor Inaba at 10 days and 3 months post-vaccination.
VAXCHORA recipients were divided into 2 cohorts, 1 challenged
at 10 days and the other at 3 months post-vaccination.
Cohort 1
Efficacy 10 days after vaccination1

In the 10-day challenge group, 2 volunteers receiving VAXCHORA (2/35, 5.7%) developed moderate to severe diarrhea after challenge with V. cholerae.1
Cohort 2
Efficacy 3 months after vaccination1

In the 3-month challenge group, 4 volunteers taking VAXCHORA (4/33, 12.1%) developed moderate to severe diarrhea after challenge withV. cholerae.1
In the case of each cohort (10-day and 3-month), evaluation of the relative efficacy of VAXCHORA was made in comparison to the combined outcomes among recipients of placebo in both cohorts.1
The majority of placebo recipients (39/66, 59.1%) developed moderate or severe diarrhea after being challenged with V. cholerae.1
- a Moderate to severe diarrhea defined as ≥3.0 L or ≥5.0 L, respectively, within 10 days after being challenged with V. cholerae 01 El Tor Inaba.3
- b Vaccine efficacy=[(Attack Rate in Placebo Group – Attack Rate in Vaccine Group)/Attack Rate in Placebo Group] x 100.
Immunogenicity (seroconversion) Trials — Studies 1, 4, and 5
A series of immunogenicity trials further contributed to the demonstration of VAXCHORA's efficacy, particularly in other age groups than were enrolled in Study 2. In these studies, immune response to VAXCHORA was based on seroconversion. A vibriocidal antibody assay was used to measure serum levels of neutralizing antibodies against the vaccine strain (classical Inaba), and seroconversion was defined as a ≥4-fold rise in serum vibriocidal antibody from baseline to 10 days post vaccination. Based on an observed association in Study 2 (the challenge study in adults aged 18-45 years) between seroconversion and protection from V. cholerae disease, seroconversion rate at 10 days post-vaccination was used to evaluate response to vaccination in these immunogenicity trials.1
Study 1 — Immunogenicity Study in Adults Aged 18-45
Study Design: Study 1 was a randomized, double-blind, saline placebo-controlled safety and immunogenicity study conducted in the US and Australia. A total of 3146 subjects 18 through 45 years of age not previously exposed to cholera were randomized 8:1 to receive 1 dose of VAXCHORA or placebo.1
Seroconversion after 10 days

Seroconversion rates (95% CI) were: vaccine recipients, 93.5% (92.5%, 94.4%); placebo, 4% (2%, 7%).1
Study 4 — Immunogenicity Study in Adults Aged 46-64
Study Design: Study 4 was a randomized, double-blind, placebo-controlled safety and immunogenicity study conducted in the US. A total of 398 subjects 46 through 64 years of age with no prior history of cholera infection or travel to a cholera-endemic area in the previous 5 years were randomized 3:1 to receive 1 dose of VAXCHORA or placebo.1
Vibriocidal antibody seroconversion rates for the classical Inaba strain were compared at 10 days post-vaccination between subjects 46 through 64 years of age in Study 4 and subjects 18 through 45 years of age in Study 1.
64 years of age1
Study 4
Subjects 46 through 64 years of age
(VAXCHORA N=291a)

Seroconversion rates (95% CI) were: Study 4, 90.4% (86.4%, 93.5%); Study 1 (subjects 18 through 45 years of age; VAXCHORA N=2687), 93.5% (92.5%, 94.4%); difference in seroconversion rates -3.1% (-6.7%, 0.4%).b
- a N represents number of subjects with analyzable samples at Day 1 and Day 11.
- b Prespecified success criterion was that the lower bound of the 2-sided 95% CI on the difference in seroconversion rate (Study 4 minus Study 1) must be greater than -10 percentage points.
Efficacy in children and adolescents
Study 5 — Immunogenicity Study in Children and Adolescents Aged 2–17
Study Design: Study 5 was a randomized, double-blind, saline placebo-controlled safety and immunogenicity study conducted in the US. A total of 550 subjects 2 through 17 years of age not previously exposed to cholera were randomized 6:1 to receive one dose of VAXCHORA or placebo. Randomization was stratified by age into 3 cohorts: Cohort 1: 12 to <18 years of age; Cohort 2: 6 to <12 years of age; Cohort 3: 2 to <6 years of age.1
Classical Inaba vibriocidal antibody seroconversion rates at 10 days post-vaccination were compared with seroconversion rates in adults 18 through 45 years of age in Study 1.
Study 5
Subjects 2 through 17 years of age
(VAXCHORA N=399a)

Seroconversion rates (98.3% CI) were: Study 5, 98.5% (96.2%, 99.4%); Study 1 (subjects 18 through 45 years of age; VAXCHORA N=2687), 93.5% (92.3%, 94.6%); difference in seroconversion rates 5.0% (2.8%, 6.4%).b
- a N represents number of subjects with analyzable samples at Day 1 and Day 11 in the immunogenicity evaluable population.
- b Prespecified success criterion was that the lower bound of the 2-sided 96.7% CI on the difference in seroconversion rate (Study 5 minus Study 1) must be greater than -10 percentage points. Co-primary criterion required the lower limit of the 98.3% CI to be ≥70% for the VAXCHORA group.
Adverse Reactions1
Adverse reactions in adults
The safety of VAXCHORA was evaluated in more than 3000 volunteers aged 18 to 64 years in 4 randomized, placebo-controlled, multicenter clinical trials.
In a pooled analysis of the 4 clinical studies, 0.6% (20/3235) of VAXCHORA recipients and 0.5% (3/562) of placebo recipients reported a serious adverse event within 6 months post-vaccination. None of these events were considered to be related to vaccination.
No serious adverse events were found to be related to vaccination.
Study 1a
Adverse reaction
VAXCHORA
(N=2789)b
Placebo (saline)
(N=350)b
- a Data are derived from Study 1 (NCT02094586).
- b N represents number of subjects who completed a memory aid.
Adverse reactions in children and adolescents
The safety of VAXCHORA in children was evaluated in a randomized, placebo-controlled, multicenter clinical trial that included a total of 543 children 2 through 17 years of age. Randomization was stratified by age, and children were enrolled in 3 separate age cohorts: 12-<18 years (Cohort 1), 6 -<12 years (Cohort 2), and 2-<6 years (Cohort 3).
The safety analysis set included 468 VAXCHORA recipients and 75 placebo recipients.
In this trial, 0.2% (1/468) of VAXCHORA recipients and 1.3% (1/75) of placebo recipients reported a serious adverse event within 6 months post-vaccination.
No serious adverse events were found to be related to vaccination.
Cohort 1
Ages 12 to <18 years
Cohort 2
Ages 6 to <12 years
Cohort 3
Ages 2 to <6 years
Adverse reaction
VAXCHORA
(N=165)b
Placebo (saline)
(N=24)b
VAXCHORA
(N=157)b
Placebo (saline)
(N=25)b
VAXCHORA
(N=146)b
Placebo (saline)
(N=26)b
Cohort 1 Ages 12 to <18 years
Adverse reaction
VAXCHORA
(N=165)b
Placebo (saline)
(N=24)b
Cohort 2 Ages 6 to <12 years
Adverse reaction
VAXCHORA
(N=157)b
Placebo (saline)
(N=25)b
Cohort 3 Ages 2 to <6 years
Adverse reaction
VAXCHORA
(N=146)b
Placebo (saline)
(N=26)b
- a Data are derived from Study 5 (NCT03220737).
- b N represents number of subjects who completed a memory aid.
In total, 13.2% of VAXCHORA recipients reported an unsolicited adverse event that was considered related to study treatment, compared with 9.3% for placebo recipients.
Dosage and Administration1
VAXCHORA helps deliver cholera protection in just 1 dose
1
DOSE
VAXCHORA is administered as a single oral dose.
10
DAYS
VAXCHORA should be administered at least 10 days before potential exposure to cholera.
4
HR
Recipients should consume VAXCHORA at room temperature in a healthcare setting within 4 hours of reconstitution. Consume VAXCHORA within
30 minutes, if sucrose or non-flavored stevia are added.
60
MIN
Recipients should not eat or drink for 60 minutes before and after ingestion of VAXCHORA.
Preparation and administration of VAXCHORA
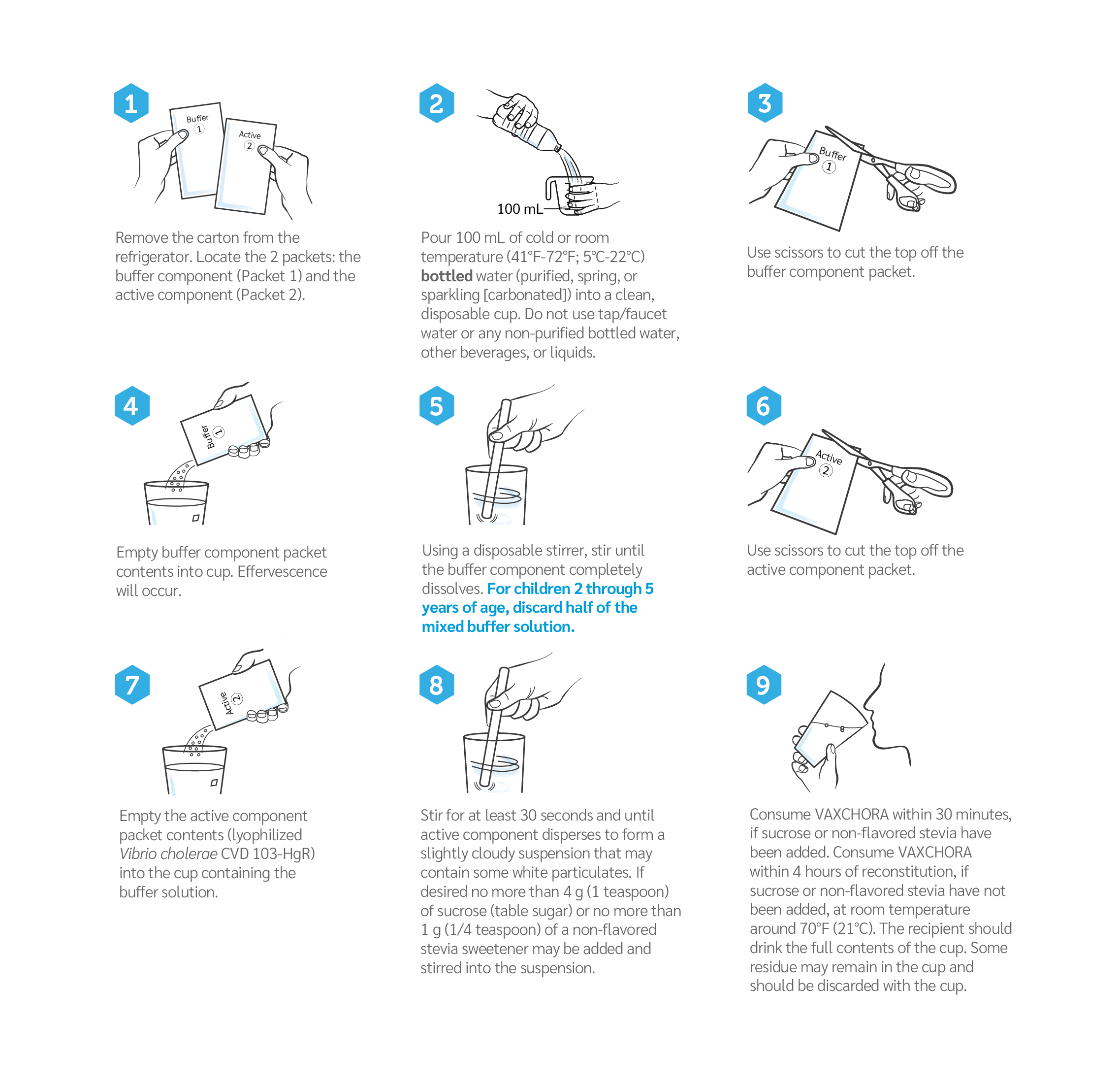
How Supplied/Storage and Handling1
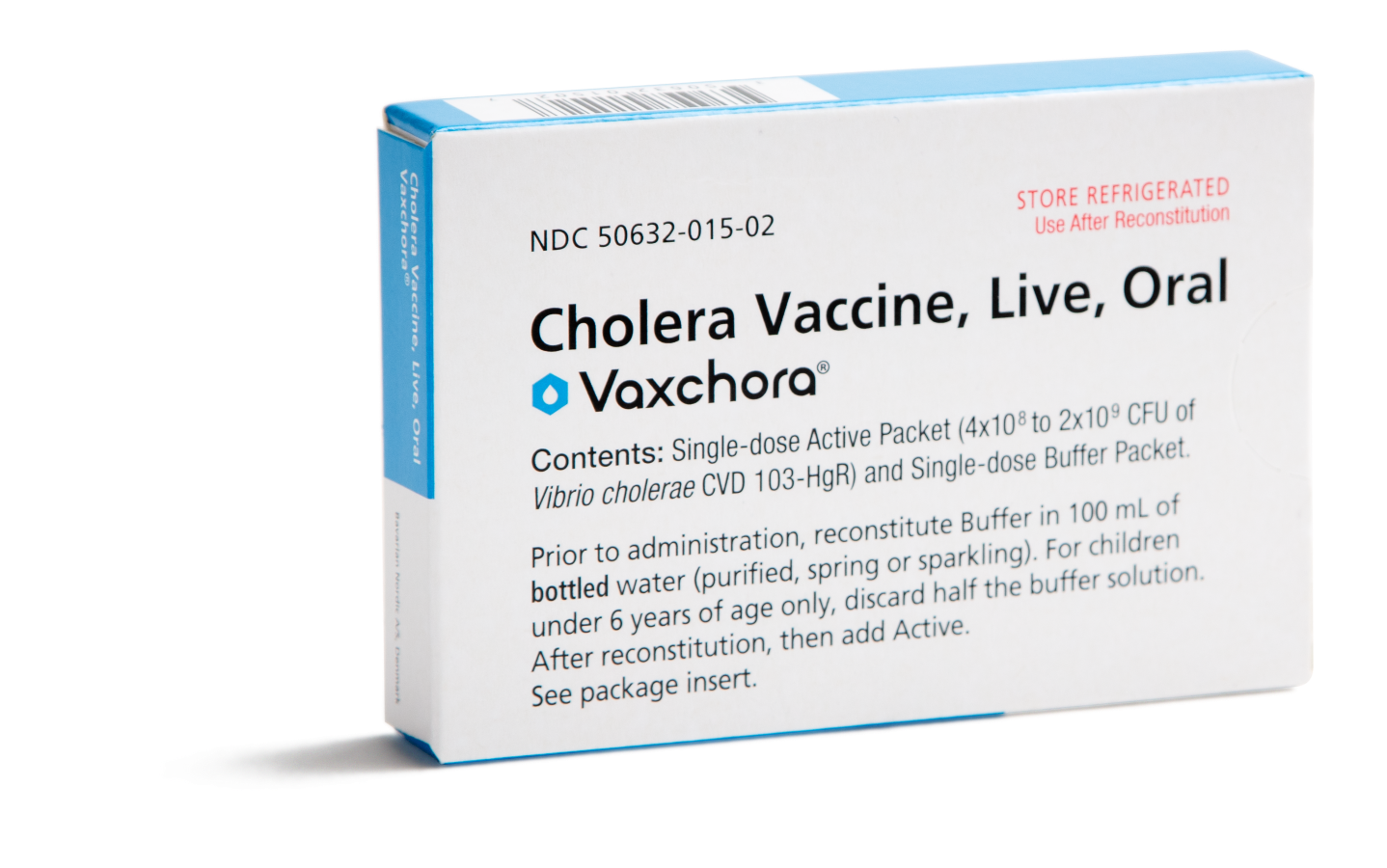
How supplied1
Single-dose carton
containing 2 packets
NDC 50632-015-02
Buffer Component Packet
NDC 50632-015-02
Active Component Packet
NDC 50632-015-02
Storage and handling1
- Store VAXCHORA buffer component and active component packets refrigerated at 36°F to 46°F (2°C to 8°C)
- Protect from light and moisture
- Packages may be stored at 48°F to 77°F (9°C to 25°C) for no more than 24 hours prior to reconstitution
- Dispose of the cup, packets, and stirrer according to standard procedures for medical waste
For medical inquiries about VAXCHORA, please contact Medical Information at
(844) 422-8274 or medical.information_na@bavarian-nordic.com.
Visit the Resources page for more information about VAXCHORA.
View NowIndication and Usage
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera-affected areas.
Limitations of Use: The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
Important Safety Information
Contraindications
VAXCHORA is contraindicated in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine.
Warnings and Precautions
Immunocompromised Persons: The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons and the immunologic response to VAXCHORA may be diminished in immunocompromised individuals.
Shedding and Transmission: Because VAXCHORA may be shed in the stool of recipients for at least 7 days and the vaccine strain can potentially be transmitted to non-vaccinated close contacts (e.g., household contacts), use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts.
Adverse Reactions
In adults 18-45 years old, the most common adverse reactions (incidence >3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%), and diarrhea (4%).
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
- Cohort 1 – age 12-<18 years: headache (45%), tiredness (41%), abdominal pain (38%), lack of appetite (29%) and nausea (22%)
- Cohort 2 – age 6-<12: tiredness (35%), abdominal pain (27%), headache (26%), lack of appetite (15%) and nausea (14%)
- Cohort 3 – age 2-<6: tiredness (31%), lack of appetite (19%), and abdominal pain (17%)
Drug Interactions
Antibiotics: Avoid concomitant administration of VAXCHORA with systemic antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination.
Antimalarial Prophylaxis: Immune responses to VAXCHORA may be diminished when administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning chloroquine.
Immunosuppressive Treatments: Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune response to VAXCHORA.
Patient Counseling
Food and Water Safety Vigilance: Vaccine recipients should be advised to exercise caution regarding food and water consumed in cholera-affected areas, in accordance with the recommendations from the Centers for Disease Control and Prevention for the prevention of cholera in travelers.
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic at 1-833-365-9596 or the US Department of Health and Human Services by either visiting www.vaers.hhs.gov/reportevent.html or calling 1-800-822-7967.
Please see full Prescribing Information.
References:
- VAXCHORA® (Cholera Vaccine, Live, Oral) [package insert]. Redwood City, CA: Emergent Travel Health Inc.
- Centers for Disease Control and Prevention. Cholera information for health care professionals. Updated August 10, 2023. Accessed January 5, 2024. https://wwwnc.cdc.gov/travel/page/cholera-travel-information
- Chen WH, Cohen MB, Kirkpatrick BD, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae 01 El Tor. Clin infect Dis. 2016;62(11):1329-1335.
Indication and Usage
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera-affected areas.
Limitations of Use: The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
Important Safety Information
Contraindications
VAXCHORA is contraindicated in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine.
Warnings and Precautions
Immunocompromised Persons: The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons and the immunologic response to VAXCHORA may be diminished in immunocompromised individuals.
Shedding and Transmission: Because VAXCHORA may be shed in the stool of recipients for at least 7 days and the vaccine strain can potentially be transmitted to non-vaccinated close contacts (e.g., household contacts), use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts.
Adverse Reactions
In adults 18-45 years old, the most common adverse reactions (incidence >3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%), and diarrhea (4%).
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
- Cohort 1 – age 12-<18 years: headache (45%), tiredness (41%), abdominal pain (38%), lack of appetite (29%) and nausea (22%)
- Cohort 2 – age 6-<12: tiredness (35%), abdominal pain (27%), headache (26%), lack of appetite (15%) and nausea (14%)
- Cohort 3 – age 2-<6: tiredness (31%), lack of appetite (19%), and abdominal pain (17%)
Drug Interactions
Antibiotics: Avoid concomitant administration of VAXCHORA with systemic antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination.
Antimalarial Prophylaxis: Immune responses to VAXCHORA may be diminished when administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning chloroquine.
Immunosuppressive Treatments: Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs, and corticosteroids (used in greater than physiologic doses), may reduce the immune response to VAXCHORA.
Patient Counseling
Food and Water Safety Vigilance: Vaccine recipients should be advised to exercise caution regarding food and water consumed in cholera-affected areas, in accordance with the recommendations from the Centers for Disease Control and Prevention for the prevention of cholera in travelers.
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic at 1-833-365-9596 or the US Department of Health and Human Services by either visiting www.vaers.hhs.gov/reportevent.html or calling 1-800-822-7967.
Please see full Prescribing Information.
